The COVID-19 crisis has reinforced the need to treat the most vulnerable patients at home. Home infusion pharmacies have been safely and effectively providing a wide range of intravenous (IV) medications to patients in their homes for more than 40 years. And, as in home nursing services, there has been a significant uptick in home infusion referrals for patients who are best served by avoiding facility-based care during and after the pandemic.
Home infusion’s proven model of care is overwhelmingly preferred by patients and is also cost effective compared to other sites of care. A 2017 study published in the journal “Healthcare” shows that up to 95% of patients who are dependent on IV medications prefer to be treated at home, and nearly 98% of patients indicated in a 2020 study in “Infusion” that they are highly satisfied with their home infusion services.
Commercial payers, Medicaid programs and other payers recognize the advantages to patients as well as substantial cost savings and have put “site of care optimization” programs in place, using tools such as benefit structuring and prior authorizations to encourage patients to choose home infusion. For Medicare beneficiaries, however, a comprehensive, straight-forward home infusion benefit does not exist. The National Home Infusion Association (NHIA) estimates that 17 million to 24 million seniors with underlying health conditions don’t have coverage for home infusion and are forced to either pay out of pocket, receive treatment in more expensive facility-based settings or skip needed medical treatments.
Reduced Utilization: a Red Flag
In the larger scheme of things, Medicare only offers home infusion benefits for a limited subset of drugs—about 30 medications that require an external infusion pump to administer. These drugs are covered under the Part B Durable Medical Equipment, Prosthetics, Orthotics and Supplies (DMEPOS) benefit. However, there are more than 100 medications—including antibiotics, hydration and monoclonal antibodies—covered under Part D that are routinely administered at home using simple methods (e.g., IV push or gravity drip). Because they do not require a pump, these drugs are not eligible for Medicare’s Part B coverage of home administration supplies and services.
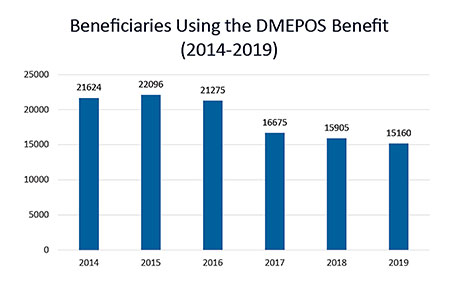
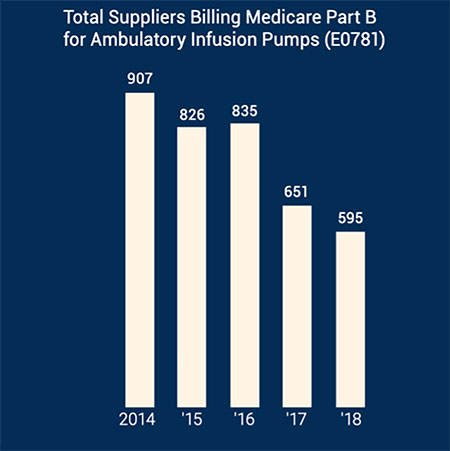
Among the medications for which home infusion services are covered, severely limited reimbursement and an improper interpretation of legislation intended to increase beneficiary access to care under the Part B benefit has actually worked to reduce the utilization of services. New data from the Centers for Medicare and Medicaid Services (CMS) shows that utilization of Part B DMEPOS infusion drugs declined 2% from 2018 to 2019 and 31.3% from a six-year high in 2015 (see Chart 1). In addition, provider participation in the program declined to its lowest point in five years (see Chart 2). All this despite a new reimbursement model that includes payment for nursing services under the home infusion transitional benefit, which went into effect Jan. 1, 2019. A permanent benefit that included some modifications went into effect Jan. 1, 2021.
These trends within Medicare contradict what is happening in the commercial market. Overall, home infusion utilization is growing, not shrinking. A report published by NHIA in 2020 shows overall industry growth exceeded 300% during the past decade, largely driven by an aging population, higher demand for home-based care and cost savings. The United States Census Bureau estimates that the 65-and-older population grew by 34% from 2010 to 2019, yet fewer beneficiaries are using the Part B home infusion benefit. NHIA interprets a drop in Medicare utilization of home infusion services as evidence of CMS’s flawed interpretation of the current law. While the Part B DME benefit is important in addressing home infusion access for a small and highly vulnerable population, the structure of the program has ultimately failed the beneficiaries it was designed to support.
Addressing the Core Issues
Congress included provisions in the 21st Century Cures Act and the Bipartisan Budget Act of 2018 to create a professional services benefit for Medicare Part B home infusion drugs. The intent in establishing this benefit was to maintain patient access to home infusion by covering professional services, including assessments; education on administration and access device care; monitoring and remote monitoring; coordination with the patient, caregivers and other health care providers; and nursing visits. Despite Congress’s intent, CMS improperly implemented the benefit by requiring a nurse to be physically present in the patient’s home for providers to be reimbursed. As a practical matter, the current home infusion therapy benefit only acknowledges face-to-face visits from a nurse and fails to account for the extensive clinical and administrative services that are provided remotely by home infusion clinicians.
The Cures Act also broadened the definition of a home infusion therapy services supplier to include physicians, home health agencies and others—in addition to infusion pharmacies. However, as of March 2021, fewer than than 250 total suppliers (including pharmacies) have enrolled nationwide. If CMS’s goal was to recruit nursing agencies to the benefit, the effort appears to be falling short. Only 41 nursing agencies in 12 states have enrolled to provide services, and 59% of the 41 are located in just three states.
NHIA believes that future beneficiary access to home infusion under Part B will depend on the DME pharmacy’s ability to secure nursing care, which can no longer overlap with Part A home health. Achieving sufficient participation in both DMEPOS home infusion and Part B home infusion therapy services to maintain beneficiary access will be challenging given the small number of potential beneficiaries and low reimbursement compared to the expense of achieving and maintaining accreditation.
To fix this situation, it is vital that reimbursement for home infusion services reflect all the services necessary to administer IV drugs safely and effectively at home—including both face-to-face services provided in the patient’s home and the extensive pharmacy services conducted behind the scenes. Consistent with the successful commercial market model, this would require reimbursement to occur every day a drug is infused, rather than just on days when a skilled professional is physically present.
The Preserving Patient Access to Home Infusion Act provides technical clarifications that will remove the physical presence requirement, ensuring payment regardless of whether a health care professional is present in the patient’s home. The legislation also acknowledges the full scope of professional services provided in home infusion—including essential pharmacist services—into the reimbursement structure.
Preliminary analysis of the legislation from the Moran Company suggests that the measure will create savings for patients and taxpayers by moving care into more cost-effective home settings. “Our model estimates on balance that the legislation would produce more savings than costs—with an estimated savings over 10 years of $93 million,” the report concludes.
The legislation does not address the coverage gap for the more than 100 home infusion medications that do not require a pump for administration. For those therapies, the Biden administration could leverage its authority under the Center for Medicare & Medicaid Innovation to establish a demonstration that would bring the same cost savings and patient benefits to Medicare that other payers have enjoyed for years. Millions of seniors that lack access to coverage for home-based care could gain from this expansion, and such a demonstration could easily leverage existing Medicare infrastructure to establish a meaningful home infusion benefit.
Conclusion
Medicare is the only major payer of health care services in the United States that lacks coverage for administering IV drugs in the home, despite an overwhelming need for patients to remain at home during the pandemic and the significant potential for cost savings. While commercial payers are modifying benefit design to incentivize beneficiaries to use home as the site of care, CMS policies have pushed beneficiaries back to facilities. It’s time for Medicare to recognize the value of home infusion for both patients and taxpayers and expand this benefit to ensure access is available for all of America’s seniors.
Connie Sullivan, BSPharm, is the president and CEO of the National Home Infusion Association (NHIA). Her efforts are focused on advocating for expanded Medicare coverage of home infusion services, advancing industry data initiatives to improve the quality of patient care and establishing educational programs to advance the technical capabilities of the industry. She can be reached at connie.sullivan@nhia.org.
